Signed in as:
filler@godaddy.com
Signed in as:
filler@godaddy.com

is the most frequent ICU acquired infection among patients receiving mechanical ventilation(1). It is estimated that the incidence of VAP in the Critical Care environment ranges between 10% and 20% of patients (2) and this results in 24% to 50% mortality(3)
The Venner PneuX(TM) System addresses multiple known risk factors associated with intubation systems in current standard use.

The PneuX(TM) System is indicated for use in Adult Critical Care for patients requiring intubation, when potential VAP can occur. Patient groups would include General Intensive Care, High-Risk Cardiac, Burns, Neuro, Colorectal and Transplant patients.
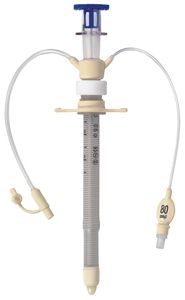
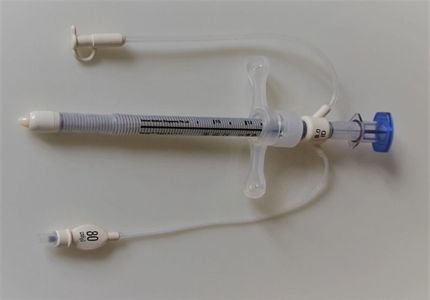
The technology comprises of a specially designed single-use silicone, flexible, low-volume low-pressure cuffed endotracheal/tracheostomy tubes and Tracheal Seal Monitor.
The secretions that gather above the ETT cuff are laden with bacteria, and are harmful to the patient if they enter the lower trachea and lungs, thus causing a VAP(1).
Whilst a normal ETT cuff will be inflated to secure a good seal for ventilation purposes, there remain folds in the cuff that allow secretions to gradually pass the cuff via these channels. Some of the newer ET tubes have been tested for microaspiration, as can be seen in the Leak test below.
The three ports just above the cuff allow the clinical team to perform Subglottic Secretion Drainage, as well as Irrigation.
Has a strong commitment to providing support and training for its products
The experienced and dedicated team at Qualitech Healthcare Ltd are able to offer in-depth training for the Venner PneuX(TM) System
It is a requirement of Qualitech Healthcare Ltd that any ICU or CICU evaluating or purchasing the Venner PneuX(TM) System is provided with full product training dispensed to key clinical and nursing staff PRIOR to the actual introduction of the product
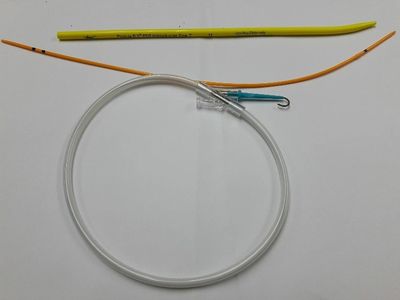
Venner PneuX™ PDT Introducer Set, an innovative advancement, intended for controlled, elective, subcricoid insertion of a tracheostomy tube in combination with compatible PDT Sets.
The Venner PneuX™ TT (as shown below), is longer than a conventional tracheostomy tube and so the longer Venner PneuX™ PDT Introducer and Wire Stiffener is required for convenient placement.
An evidence-based, multifactorial approach for the prevention of Ventilator-Associated Pneumonia (VAP)
· Indicated for use in adult critical care for patients requiring intubation (primary or tube exchange) and mechanical ventilation
· Specifically designed silicone, low-volume, low-pressure (LVLP) tracheal tube/cuff
· Prevents pulmonary aspiration (1)
· Permits intermittent subglottic secretion drainage and irrigation - three subglottic ports
· Protects the tracheal wall – the Venner PneuX TSM™ Cuff Pressure Controller maintains a constant cuff/tracheal wall seal pressure preventing aspiration (1).
(1) Mariyaselvam et al. BMC Anaesthesiology 2017;17:36

This website uses cookies. By continuing to use this site, you accept our use of cookies.